Transvaginal Mesh Lawyers
Serious Complications Associated with the Use of TransVaginal Surgical Mesh Devices
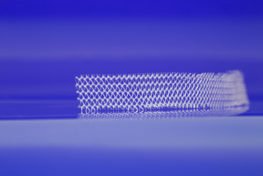
A TransVaginal Mesh surgical device is primarily used to treat conditions such as stress urinary incontinence, pelvic organ prolapse and other similar issues. Specifically, the mesh itself is made from a porous woven fabric that is implanted in the vagina in order to create a sling for prolapsed organs or, to reinforce the vaginal wall.
While the surgical mesh was certainly intended to benefit women who are suffering from urinary and gynecological warranting its implantation, more than 15% of women who received the surgical mesh have reported serious complications, such as:
- Erosion and/or migration of the mesh material
- Chronic pain
- Infections
- Painful intercourse
- Perforation of the pelvic organs and bladder
- Urinary complications
- Reoccurring organ prolapse
- Scarring and/or shrinkage of the vaginal wall
FDA Warnings about the Use of TransVaginal Mesh Devices
On July 13, 2011, the FDA issued an updated warning regarding the use of surgical mesh devices, targeting surgeons, doctors, and patients who have already or are considering having the mesh implantation procedure. Importantly the 2011 warning updates the original one issued on Oct. 20, 2008, addressing the serious medical complications associated with having the mesh surgery and also, clarifying that said complications, especially in cases involving pelvic prolapse, are not rare as had previously been thought.
A TransVaginal Mesh May Not Be Necessary
According to FDA scientific literature, people who undergo TransVaginal Mesh surgery are exposed to more serious health risks in comparison to those that simply receive stitches to repair a pelvic organ prolapse. While a surgical mesh can certainly correct certain anatomical issues, there is no evidence to suggest that a mesh provides any greater medical benefit than non-mesh surgical alternatives. In the case of stress urinary incontinence, the FDA is still evaluating whether a vaginal mesh is effective in treating this condition.
TransVaginal Mesh in the News
Recently, a woman in California received $5.5 million in monetary damages from a vaginal mesh manufacturer due to complications associated with mesh implantation surgery. The verdict specifically found that the manufacturer, namely C.R. Bard, Inc., negligently handled and marketed its vaginal mesh device, as well as improperly tested the device before it was released into the market. This case is the first of many that will be tried against manufacturers of TransVaginal mesh devices.
Due to the increasing number of vaginal mesh lawsuits being filed against manufacturers across the country, Johnson & Johnson (J&J) announced a few weeks back that it was going to stop selling the mesh implants. J&J is currently facing thousands of lawsuits filed against it for complications due to the devices, and as such, requested that the FDA allow them to cease selling four of its vaginal mesh devices, over a four month period.
Why Hire an Attorney?
It is crucial to speak with an attorney who is qualified to handle the various complexities and nuances associated with suing device manufacturers. Only skilled lawyers can help victims of TransVaginal Mesh devices recover monetary damages for costs due to medical treatment, pain and suffering, as well as lost wages. You certainly need a product liability attorney on your side who has the experience necessary to help you get the compensation that you deserve.
Vaginal Mesh Case Evaluation
Our law firm represents each client with care and compassion. At Stern Law, we believe “It’s always about the client first.”
Give us a call today to see if you or a loved one may be entitled to recover monetary damages from complications caused by TransVaginal Mesh manufacturers. Call attorney Ken Stern at 1-844-808-7529, or complete an online contact form for assistance.













